
Chapter 7
Daguerreotypes, Ambrotypes, and Tintypes
This chapter discusses Daguerreotypes, tintypes, ambrotypes, and ambrotype derivatives Hallotypes, Diaphanotypes, spherotypes, and alabastrines.
Specimens of Daguerreotypes, ambrotypes, and tintypes are
sometimes mistaken for each other in similar decorative cases.
Daguerreotypes and ambrotypes were always cased; only tintypes
were both cased and uncased. When cased, tintypes resemble
ambrotypes on cursory inspection. The normally rather obvious
differences in the three types are often obscured by
deterioration and by original process variations. Unlike paper
photographs, however, these three types did not fade. It took
many years to recognize and control impurities in paper and
gelatin, and in processing chemicals.
Daguerreotypes
The literature on Daguerreotypes is phenomenal in physical
volume and in the vitality of modern research. Virtually all
photographic history books contain accounts of the invention
and worldwide acceptance of the process from about 1840 to the
mid 1860's. The calotype made only minor inroads in its
popularity, even though the calotype negative permitted
duplication, while the Daguerreotype had to be rephotographed
or etched and inkprinted. The wet collodion and tintype
processes finally superseded the Daguerreotype, but it left a
rich legacy of some of the earliest historical photographic
images.
Besides the standard history books, Gernsheim [61], Barger [8],
and Newhall [104] have separate histories of the Daguerreotype,
based on historical and cultural factors. The process has been
revived in recent years, notably by Irving Pobboravsky of the
Rochester Institute of Technology, with beautiful results.
Romer [126] estimates that there are or have been several dozen
modern practitioners of the art.
The Daguerreotype has been studied more extensively by modern
analytical methods then any other historical photographic
process. Most of the results to date are listed in the
bibliography under Modern Scientific Studies. The definitive
work has been reported by M. Susan Barger and her collaborators
[references 7 through 18]. In particular, Barger and White,
reference 15, is a work of major significance, not only
regarding the Daguerreotype but also parallel branches of
photography in that period. Other work is by Pobboravsky [118
and [119], Swan et al [138], and Jacobson & Leyshon [80]. A
scientific model is described by Barger [8 and 12]. Modern
scientific interest in the process is aroused by its embodiment
of thin film physics and optics. It is the only completely
inorganic chemical photographic system with no emulsion, which
makes it an interesting model for photosensitive research.
Daguerreotypes are probably the easiest of the three cased
types to identify because the polished silver exhibits specular
reflection. This means that they are silver mirrors in which
the viewer can see a true image reflected, not just a metallic
sheen. The appearance depends critically on the viewing
angle.
The nature of the Daguerreotype image is shown in scanning
electron micrographs in Appendix I. Highlights in the image
contain a high density of light - scattering amalgam particles,
so that some incident light has a good probability of reaching
the viewer's eye. Shadows have fewer such particles, so
incident light is efficiently reflected away from the eye
unless the viewing angle is very close to ninety degrees. In
the latter case, the viewer will see his or her own image.
The polished silver is a property unique to Daguerreotypes and
a valuable aid to recognition, but there are two problems.
First, the silver is subject to tarnishing, especially around
the edges as shown in Figure 5. Second, all Daguerreotypes have
protective glass over the picture, and reflections from the
glass can be mistaken for reflections from the silver. This may
confuse identification because all ambrotypes and some tintypes
were also glass covered.

|
| Figure 5 |
Daguerreotypes were made in standard sizes (not all
authorities agree on these sizes):
|
Table 2
|
|
| Whole plate | 6-1/2 x 8-1/2 inches |
| Half plate | 4-1/4 x 5-1/2 |
| Quarter plate | 3-1/4 x 4-1/4 |
| Sixth plate | 2-3/4 x 3-1/4 |
| Ninth plate | 2 x 2-1/2 |
| Sixteenth plate | 1-3/8 x 1-5/8 |
In addition, there was a "double whole plate", also called
Mammouth or Imperial plate, 10 1/2 x 13 1/2 inches. This was
the largest Daguerreotype size ever made, and a few were made
about 1850. According to Condax [35] no camera capable of
holding these plates is known to exist today.
Most Daguerreotypists bought whole plates and cut them to
desired sizes, using much ingenuity to minimize waste. Rough
cut edges and corners are common, concealed in the cases. Blank
plates were supplied to the trade, mostly from French and
American sources, and were made by two processes: (1)
electroplated silver on copper, and (2) cladding.
Cladding was discovered about 1742 by Thomas Boulsover. It is a
process of fusion bonding by alloying a bar of silver against a
bar of copper and running them together through a rolling mill
under great pressure. The process is described in Bisbee [23].
The silver thickness of clad plates was one-fortieth to
one-sixtieth of the copper thickness; the number 40 was often
stamped in one corner of whole plates. Clad plates were used
for the earlier Daguerreotypes, while electroplated plates were
later used by some Daguerreotypists.
Electroplating was patented in 1840 and put into practical use
about 1844; it depended on the availability of electric
current. 'Galvanic' batteries were used as a power source, and
electroplated plates were called 'galvanized' (modern usage of
the term refers to hot-zinc dipping). Pobboravsky [119, 42]
states that French electroplated Daguerreotype plates were made
as early as 1851, with an embossed hallmark of the process.
The microstructure of the silver surface is different in the
two processes. Rolling generates minute longitudinal marks,
while electroplating produces a more porous grain structure
which can be seen microscopically. Fusion bonding also produces
some alloying of the copper in the silver, which varied with
process parameters. In principle it should be possible to trace
the source of a plate by analysis of these characteristics.
Both processes are common metallurgical operations today.
The sensitized plates were exposed directly in the camera,
generating a reversed image (see Chapter 11), but not quite all
Daguerreotypes are reversed. Some are rephotographed copies, so
that shop signs, for example, read normally. Others were made
by photographing through a 45 degree prism mounted in front of
the camera lens, or from a mirror. Both of these techniques
produced a normal picture but were not often used because they
were too much trouble and expense. People were so entranced
with the novelty of fixed images that it didn't really matter
if portraits were reversed.
There have been several published processes in the past few
years for removing tarnish from Daguerreotypes. It is strongly
recommended that none of them be used without first reviewing
the most recent techniques: see the comments in Chapter 12 and
Appendix I. Cosmetic reasons are not sufficient to justify the
risk of irreversible loss of image information.
Ambrotypes
Ambrotypes were more popular in America, appearing from 1854
until about 1865; their European name was amphitype. They are
collodion negatives (not positives) on glass, sandwiched
against dark background materials in a case. They appear as
positives for the following reason. When any transparent silver
based negative is viewed from either side, a small amount of
light is reflected back to the viewer from the shadows;
essentially no light is reflected from the highlights. This is
difficult to verify in a brightly lighted room because so much
light comes through the negative, but it can be seen in a
darkened room with the illumination coming from behind the
viewer. If a matte black surface is placed behind the negative,
it will prevent any light from coming back to the viewer from
the clear regions, transforming them into shadows. Light will
still be reflected from the darkened areas of the negative and
they become highlights relative to the clear areas. Thus the
negative now appears as a positive, though not very bright or
contrasty by modern standards. Daguerreotypes were usually not
very contrasty either, so ambrotypes became competitive,
especially since they were cheaper.
Ambrotypes often look like they were made on a dark and stormy
night. Efforts were made to improve the contrast; exposure and
development techniques were optimized, and tinting helped to
relieve the dullness. Different kinds of background were used;
japanned black cardboard, velvet, black varnished metal, and
black varnish applied directly to the collodion negative.
Towler [108, 138] lists four varnish formulations that could be
applied to either side of the glass negative. If it was applied
to the collodion side the picture was not reversed to the
viewer but it was duller than if the glass on the side opposite
the collodion was varnished. Most ambrotypes are reversed as a
tradeoff for a slightly brighter appearance.
Varnish on the glass is often blistered after a century and a
quarter; in such cases the picture appears hideous and
apparently worthless, but there is hope of restoration. The
picture can be restored by removing the old lacquer; this is a
task for a skilled restorer who knows which solvent will remove
the varnish and not the collodion picture. Black paper
(acid-free archival quality) will then restore the picture if
the collodion image is intact. Sometimes just placing black
paper against the blistered varnish will improve the
appearance, but it is not a proper restoration and it may
abrade the collodion if that is the side that was varnished.
Neither Daguerreotypes nor tintypes show this particular form
of deterioration, so blistering is at least an aid to
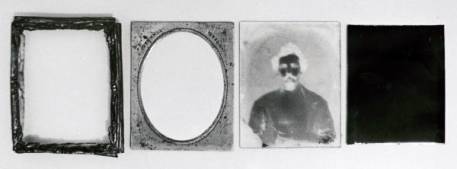
identification. Figure 6 shows an the component parts of an
ambrotype that is backed with a piece of black lacquered iron
with formed raised edges to prevent close contact with the
glass. The collodion surface can thus face the backing without
abrasion damage, and the picture is not reversed. This backing
has survived without deterioration. The image photographed on a
white background can be seen to be a negative.
|
| Figure 6 |

|

|
| Figure
7a |
Figure 7b |
Note that in paper processes, shadows (not highlights) are
rendered by heavy silver deposits in the negative, and positive
prints are produced in a second step from negatives. Highlights
in paper prints derive their color from the underlying paper
stock.
Some tintypes are very dark overall while other specimens have
surprisingly good contrast with an almost white background that
is independent of viewing angle. Crawford [38, 43] mentions
that a grayish white background could be created by adding
mercuric chloride or nitric acid to the developer. Neither Eder
nor Towler mention this process, but there are striking
variations in the contrast range of different specimens, for
which we have been unable to establish a date correlation. The
tricks used by individual practitioners often interfere with
hopes of finding a convenient historical progression for
dating.
Tintype plates, like Daguerreotypes, were exposed directly in
the camera and therefore were reversed, but again there are
exceptions. In addition to copying, and the use of prisms or
mirrors, the collodion image could be transferred to another
metal plate. The resulting picture was called, naturally, a
transferotype and was rereversed, or normal. Further, the final
metal base did not have to be japanned iron and the magnet test
fails if it is, for example, copper or brass. These exceptions
are relatively uncommon (we have no frequency data), but the
serious historian should be aware of the possibilities.
Estabrooke's book [51] contains inserted 'non-reversed'
tintypes "made by the identical processes offered in this
book", but he fails to describe the 'non-reversal' process.
However, he describes the 'copy stand' in his darkroom and it
can be inferred that it was used. If he had used a prism at the
camera lens (see Chapter 11), one would have expected him to
mention it in his detailed description of his 'glass room', or
studio.
The collodion surface of tintypes often shows fine crazing or
cracking, which distinguishes them from ambrotypes. Remarkably,
many tintypes show no trace of rust in spite of bends and
scratches. At one time it was fashionable to adorn tombstones
with tintypes, and a few have survived a century of outdoor
exposure.
Tintypes were made in many sizes with little standardization.
The largest was 6-1/2 x 8-1/2 inches. The base material was
cheap and many tintypes are very roughcut and irregular. Some
were mounted in Daguerreotype or ambrotype cases; they can
usually be identified with a small magnet. Tintypes were often
glued on small paper mounts or mounted as cartes-de-visite. The
tiny Gem tintypes (1 x 1 3/8 inch - see Figure 8) were
sometimes mounted in stamped brass frames that resembled
Daguerreotype frames; these frames were then crimped on
cardboard mounts. But the majority of tintypes were simply
unmounted; in this form they could be mailed easily and
cheaply, making them popular during the Civil War.
 |

|
| Figure 8 |
Many tintypes are rather grubby in appearance, as Crawford
aptly describes them, and art critics universally turned up
their noses. Aesthetically they were no match for the elegant
platinotype. But they are durable and unfaded after more than a
century, and today they remain a plentiful legacy of the
appearance of Civil War soldiers, celebrities, period clothing,
and architecture.
Direct Positives
Daguerreotypes and tintypes were direct positives and were
commercially very successful, even though they lacked an
intermediate negative for reproduction. Many inventors strove
for the simplicity of single step positive processes and there
were some successes. But why does a light-struck area of the
sensitive surface appear light after processing in spite of the
earliest observations that silver salts darken when exposed to
light?
In both tintypes and Daguerreotypes, the light from highlights
in the subject produces a chemical change in the sensitive
surface. In the Daguerreotype, nucleation centers in the
highlights are converted to dense concentrations of
mercury-silver amalgam particles. These particles scatter more
reflected light to the viewing eye than does the surrounding
area with no particles, resulting in a "positive" image. In the
tintype there are no amalgam particles, but the reduced silver
particles in the highlights are more reflective than the dark
backing without silver particles exposed in the shadows. Both
processes relied upon the difference between reflectivity from
the highlights and from the shadows: there was a better chance
of light reaching the viewer from the highlights than from the
shadows.
Neither process worked on white paper, and both processes were
marginal in their contrast control compared with modern
processes.
Japanning
A description of japanning is in order, since it is rarely
described in photographic histories. Perry [111, 18] has a
useful description. Essentially it consists of baked lacquer,
usually applied in multiple layers to sheet iron, and baked
between each coat. The composition of early lacquers was
sometimes a trade secret, but Estabrooke's formula is simply
asphaltum (tar) in linseed oil. Tar is available from many
sources in nature, with variations in impurities, and japanning
quality was no doubt correspondingly variable. In Europe
japanning dated to the early 17th century, and in the East much
earlier. The original motivation was decoration, but it also
formed a very durable and rust-resistant coating that compares
favorably with some of our modern polymers.
Collodion images were sometimes printed or transferred (these
were two separate processes) on to japanned cardboard or
leather. In these cases the finish was air dried black varnish;
Towler [145, 150] has a simple recipe. True japanning requires
high temperature baking cycles that could not be used on
flammable materials, but many black varnishes or lacquers
acquired the generic term of japanning. For restoration
purposes it is not safe to assume resistance to any particular
solvent.
Japanned lacquer was produced in various colors besides black;
only the "chocolate" plate, patented in 1870, became as popular
as the black, and there are many surviving brown specimens. The
brown color was thought to be more lifelike; the same thinking
may have accounted for the popularity of sepia paper prints.
But gold or sepia toning was widely used on paper prints to
combat fading, so public acceptance of brown may have been a
factor. Tintypes and ambrotypes did not fade unless they were
grossly underfixed or washed, whereas paper prints suffered
chronically from fading problems for many years.
Tintype Nomenclature
Tintypes, the name most often used today, were also called
Ferrotypes, Melainotypes, Melanotypes, Melaneotypes,
Ferrographs, Adamanteans, Adamantines, and several other trade
names (see Estabrooke, [51]. These names reflect minor trade
differences, but they are all collodion-silver images on
japanned iron. The evolution of the many trade names is
complicated and illustrates the problems of assigning a single
identity to what now appears to us a single process. The
following account is largely paraphrased from Estabrooke's 1872
book [51].
Smith's invention is usually dated as 1854, but the date of
publication of his patent is February 19, 1856. Smith called it
the Melainotype, and Estabrooke says it was based on a French
invention of a black enamelled plate "for photographic
purposes" called the Melanotype plate. Apparently Smith's
contribution was to coat the Melanotype plate with collodion
containing a solution of silver salts.
Peter Neff bought Smith's Melainotype patent in 1856 (Eder says
1857) and continued manufacture for several years. At about the
same time (1856) Mr. V. M. Griswold of Peekskill New York
introduced his Ferrotype plates in defiance of Smith's (now
Neff's) patent. By this time the market was a free-for-all of
competing processes and tradenames, and one writer, in disgust,
referred to the various processes as 'hum-bug-otypes'. This
same writer favored the Melaneotype (sic), adding a new name to
the confusion. Some other tradenames were Adamantean, Phoenix,
Vernix, Eureka, Excelsior, Union, Star Ferrotype - all
collodion silver on japanned iron. Finally in 1870 the Phoenix
Plate Company introduced the "chocolate" plate which was a
sensation, short lived because the advent of chlorobromide
paper was imminent. Estabrooke remarks that "...in those times
every unimportant change was called a new process."
Mr. Griswold issued a rather plaintive statement concerning the
many trade names:
"Many other names have been given to similar plates, such as
Adamantine, Diamond, Eureka, Union, Vernis, Star Ferrotype,
Excelsior, and others, among which the most senseless and
meaningless is 'Tintype'. Not a particle of tin, in any shape,
is used in making or preparing the plates, or in making the
pictures, or has any connection with them anywhere, unless it
be, perhaps, the 'tin' which goes into the happy operator's
pocket after the successful completion of his work. None of
these names, however, have been considered so apt and
appropriate as Ferrotype, and it will, doubtless, be generally
accepted as long as the pictures are known." Alas, Mr.
Griswold, for your optimism.